Guideline for Diagnostic Evaluation in AAG
Enviado: Janeiro 20, 2011, 9:43 am
From The British Journal of Dermatology
Guideline for Diagnostic Evaluation in Androgenetic Alopecia in Men, Women and Adolescents
U. Blume-Peytavi; A. Blumeyer; A. Tosti; A. Finner; V. Marmol; M. Trakatelli; P. Reygagne; A. Messenger
Posted: 01/12/2011; The British Journal of Dermatology. 2011;164(1):5-15. © 2011 Blackwell Publishing
Abstract and Introduction
Abstract
Androgenetic alopecia (AGA) is the most common hair loss disorder, affecting both men and women. Due to the frequency and the often significant impairment of life perceived by the affected patients, competent advice, diagnosis and treatment is particularly important. As evidence-based guidelines on hair disorders are rare, a European consensus group was constituted to develop guidelines for the diagnostic evaluation and treatment of AGA. This S1 guideline for diagnostic evaluation of AGA in men, women and adolescents reviews the definition of AGA and presents expert opinion-based recommendations for sex-dependent steps in the diagnostic procedure.
Introduction
Evidence-based guidelines on hair disorders are rare, except for one S2 guideline on alopecia areata by the British Association of Dermatologists.[1] No national, European or international guidelines have been established for the diagnosis and treatment of androgenetic alopecia (AGA). Three different types of evidence-based guidelines (types S1–S3) exist. An S1 guideline is built by an informal consensus of an expert group. The statements for S2 guidelines are formed by a formal consensus process. An S3 guideline is based on a consensus from a systematic literature research with evaluation of evidence levels and a systematic decision process.
A European consensus group was built, consisting of members from different countries, organizations, specialities and interest groups. A detailed literature search on diagnosis and treatment of AGA using Medline, Embase, Cochrane and a hand search was performed. Based on the literature available the group decided to undergo an informal consensus process on an S1 level for the diagnosis of AGA and an S3 guideline process for the therapy of AGA (published separately). A subgroup of this European Consensus Group consisting of the eight authors of this article decided to work on this S1 guideline which was funded by the Verein Pro Haut e.V. Berlin, and is therefore independent and without any commercial conflict of interest.
The European Consensus Group reviewed the definition of AGA and established a consensus for the diagnosis of AGA dealing with the following points: expert opinion-based recommendation for the diagnosis of AGA in female and male patients, as well as in adolescents. The aim was to develop a diagnostic evaluation form and recommendations for diagnostic procedures to assist in the daily work of the practitioner. The questionnaire for daily practice was kept simple and it is planned to be validated during consultation by practising dermatologists experienced in the management of hair disorders.
Definition of Androgenetic Alopecia
AGA is a nonscarring progressive miniaturization of the hair follicle with a usually characteristic pattern distribution in genetically predisposed men and women. In men, AGA typically shows a pattern distribution, most commonly a male pattern, but occasionally a female pattern can be seen.[2,3] In women, AGA typically presents as a diffuse reduction in hair density over the frontal and central areas, but the parietal and occipital regions may also be involved.[4] Occasionally, AGA occurs in a male pattern in women.
Differential Diagnoses and Aggravating Factors of Androgenetic Alopecia
The diagnosis of AGA is normally made clinically, but aggravating associated factors and other diseases affecting scalp and hair growth need to be excluded. As certain treatments (e.g. finasteride) are selectively effective in AGA, it is important to rule out other hair loss disorders resembling pattern hair loss.
Frequency and Prevalence
Men
Male AGA occurs in all populations. The prevalence is highest in Caucasians, reaching around 80% in men aged over 70 years.[5,6] In the Asian population, a prevalence of 46·9–60·0% has been reported in males older than 70 years.[3,7] There is scant published information on the frequency of balding in African men. One older study reported that balding is four times less common in African-American men than in Caucasians. The frequency and severity of male AGA increase with age in all ethnic groups.[2,3] Initial signs of AGA, including some recession of the frontal hair line and at the temples, usually develop during teenage years. Progression to deep frontal recession and/or vertex balding may also start shortly after puberty, although in most men the onset is later. By the age of 70 years, 50–60% of Caucasian men are bald (Hamilton–Norwood VI–VII).[2,6]
Women
As in men, the population frequency and severity of AGA increase with age in women.[5] Two studies in Caucasian women in the U.K. and the U.S.A. reported prevalence rates of 3–6% in women aged under 30, increasing to 29–42% in women aged 70 years and over.[8,9] The frequency is lower in Oriental women compared with those of European descent.[3] There are no published data on the frequency of AGA in African women.
Aetiology
Men
AGA is an androgen-dependent trait, which leads to progressive miniaturization of the hair follicle in predisposed men.[10] It is thought that enhanced androgen effects at the genetically predisposed hair follicles are mediated by raised androgen receptor density and/or increased activity of 5-alpha-reductase type II.[2] Nearly all men afflicted with AGA have normal circulating androgen levels. The predisposition to AGA is predominately due to genetic factors. Twin studies show strong concordance rates of between 80% and 90% for monozygotic twins. Family analyses show a significantly increased risk for the development of AGA in men with a father suffering from AGA.[11,12] Conversely, the risk of AGA is significantly decreased in those men with a nonbalding father.
The current scientific data support the thesis that AGA has a polygenic trait. Significant associations have been reported with variant regions of the androgen receptor gene, which is located on the X-chromosome, using a candidate gene approach.[13,14] More recently, two independent genome-wide association studies have identified a susceptibility locus at chromosome 20p11.[15,16]
Women
There are few studies on the genetic base of AGA in women. Smith and Wells[12] reported the frequency of female pattern hair loss with an incidence of 54% pattern hair loss in first-degree male relatives aged > 30 years and 21% in first-degree female relatives > 30 years. It is possible that early- and late-onset female AGA are genetically distinct entities. The role of androgens in female AGA is less certain than in men and other factors may be involved. For the purpose of this guideline the term 'androgenetic alopecia' is used, although the uncertainty of the role of androgens is recognized.
The European Consensus Group agreed not to differentiate between androgenic alopecia and AGA, but to define a subset of women with AGA and associated hormonal dysregulation.
Clinical Picture
Men
In most men, AGA involves the fronto temporal area and the vertex, following a pattern corresponding to the Hamilton–Norwood scale (Fig. 1).[17,18] In some instances, however, men develop diffuse thinning of the crown with retention of the frontal hairline with a pattern that resembles the Ludwig type observed in women.
Women
Female pattern hair loss may have three different patterns:
1.Diffuse thinning of the crown region with preservation of the frontal hairline. There are two scales that describe this pattern: the commonly used 3-point Ludwig scale (Fig. 2)[19,20] and the 5-point Sinclair scale (Fig. 3).[21–23]
2.Thinning and widening of the central part of the scalp with breach of frontal hairline (Olsen scale: Christmas tree pattern, Fig. 4).[20,24]
3.Thinning associated with bitemporal recession (Hamilton–Norwood type, Fig. 1).[17,18,24]
However, involvement of the parietal and occipital scalp with diffuse alopecia is clinically observed (Fig. 5).[24,25]
Steps in the Diagnostic Procedure
The European Consensus Group developed a diagnostic evaluation form (Table 1) for AGA, including history, clinical evaluation, diagnostic techniques and clinical documentation. This form can easily be implemented in daily clinical routine. Additionally, the information has been summarized in a clinical algorithm (Fig. 6).
DD, differential diagnosis; DHEAS, dehydroepiandrosterone sulphate; FAI, free androgen index test; RPR, rapid plasma regain; SHBG, sex hormone-binding globulin; TPHA, treponemal haemagglutinin assay; TSH, thyroid-stimulating hormone.
Figure 6. Clinical algorithm for the diagnosis of androgenetic alopecia (AGA). DD, differential diagnosis.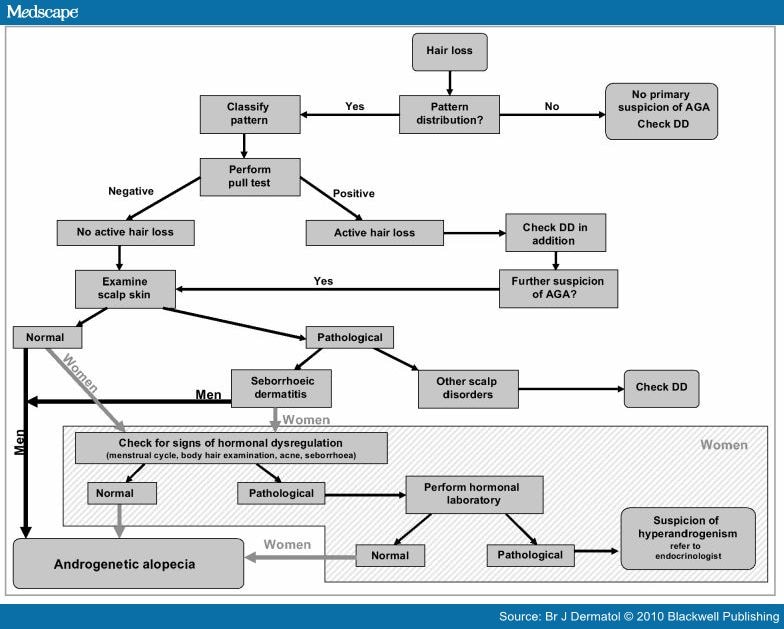 History
History
General The physician should record age, sex, age at first manifestation of hair loss and course of hair loss (chronic or intermittent) in the personal as well as in the family history. Patients with AGA usually complain about a long-standing, slowly progressing hair loss. The hair loss may be described as chronic, but the patients often report increased activity in autumn and winter. The patient should be asked about increased shedding or thinning or both. For thinning, the patient typically describes an accentuation of the frontal, parietal or vertex region, but complaints about diffuse thinning are possible as well. Initial signs of AGA can be itching and trichodynia. A family history for AGA is often positive, but a negative family history does not exclude the diagnosis. A positive family history of other hair disorders, such as alopecia areata or hirsutism may influence the extent of further diagnostic procedures. It is also important to determine if there are other concomitant hair disorders whose untreated presence could ultimately affect the efficacy of the treatments used for AGA (e.g. iron deficiency in diffuse hair loss in women).
A detailed history on systemic and newly diagnosed diseases within 1 year prior to first signs of hair loss should arouse suspicion that the hair loss is due to other causes or aggravating factors, such as diffuse effluvium as a result of severe infection, iron deficiency or thyroid dysfunction. Allergies are not important for the diagnosis of AGA, but they should be recorded for possible significance with regard to therapy (e.g. contact dermatitis due to propylene glycol in minoxidil solutions). The Consensus Group agreed to ask about eating behaviour, as chronic deficient diet or rapid significant weight loss can trigger diffuse effluvium. Concerning drug history there are a variety of drugs that can cause hair loss. Ask especially for chemotherapeutic agents (almost all of which can produce hair loss) and for hormones with pro androgenic or antithyroid action, and whether there is coincidence with hair loss. Particularly in men, the intake of anabolic steroids or supplemental androgens should be explored.
Moreover, consider lifestyle procedures such as special hairstyles (traction) and environmental factors like smoking and ultraviolet radiation (UVR) exposure in the history of the patient. Su and Chen[26] reported a dose-dependent association between smoking status and development of moderate to severe AGA in male patients. Additionally, UVR exposure as an aggravating factor should be taken into account, especially in Mediterranean countries.[27,28]
Women Although the majority of female patients with AGA have physiological hormonal function, an orienting gynaecological history should be done to exclude influencing hormonal dysregulations or associated underlying disorders (e.g. hormone-sensitive tumour). Careful evaluation of the gynaecological history should include menarche, menstrual cycle (regular/irregular), menopause, amenorrhoea, the use of oral or systemic hormonal contraception, hormone replacement therapy, fertility treatment, the number of pregnancies and births, time of last pregnancy/delivery, impaired fertility or problems in getting pregnant, miscarriages, gynaecological surgery, signs of hyperandrogenism (excessive body hair growth, skin changes like acne) or seborrhoea of scalp/skin. Irregular menstrual cycle, amenorrhoea, impaired fertility, problems in getting pregnant, miscarriages, signs of hyperandrogenism and seborrhoea may be indicative for hormonal dysregulation, such as polycystic ovary syndrome.[29–31] If the patient is taking hormonal contraception, ask for cycle duration and regularity before the intake of hormonal contraception.
Adolescents In adolescents it is first important to differentiate if the hair loss is congenital or acquired. Look for physical and mental development, particularly early onset of puberty. In childhood/adolescence, AGA is an acquired hair loss with a characteristic pattern distribution. The differential diagnosis includes diffuse effluvium (nutritional factors), artificially induced alopecia, hypotrichosis simplex (congenital) or ectodermal dysplasia (congenital, delayed physical/psychological development, sweating, dental or nail abnormalities). AGA without signs of premature puberty is not necessarily associated with endocrinological dysregulation, but referral to a paediatric endocrinologist is recommended.
Clinical Evaluation
Clinical examination should involve the scalp skin and hair, facial and body hair and skin as well as the nails.
Scalp Examination The scalp skin usually appears normal in AGA, but consider that seborrhoeic dermatitis of the scalp can be an associated and potentially aggravating factor.[6,32,33]
Look for signs of inflammation (erythema, scaling and hyperkeratosis), seborrhoea and signs of scarring (atrophy, loss of hair follicle ostia). Consider inflammatory or infectious diseases and tumours. Consider alopecia areata and scarring alopecias, which can mimic AGA, especially frontal fibrosing alopecia (Kossard[34]) in women. Look for signs of sun damage in balding areas, which may be an aggravating factor for AGA.[28] In long-standing AGA, discrete atrophy of scalp skin can be present.
Hair Examination Scalp hair Part the hair to assess scalp hair density. Compare part width in the frontal, occipital and temporal regions to examine the distribution of alopecia. On close examination, focal atrichia of a few millimetres in diameter may be present in some women with AGA. Use a sheet of paper over a part to enhance contrast and look for short and fine miniaturized or broken hairs, variation of hair calibre, length and regrowth. Dermoscopy may be helpful.[35] A pull test, Sabouraud manoeuvre and a friction test are simple tests to get a first global impression of hair quality and hair growth activity.[36,37]
Evaluate and distinguish between diffuse decrease in hair density, a characteristic pattern distribution or localized alopecic patches. In males, AGA typically presents with a male pattern distribution including bitemporal recession and/or vertex thinning, and sometimes anterior recession. About 10% of men with AGA present a female pattern.
Women usually show a more diffuse distribution of hair loss with accentuation in the frontal and mid scalp and preservation of the frontal hairline, but the parietal and occipital scalp may also be involved. In cases of diffuse thinning, also think of diffuse telogen effluvium and diffuse alopecia areata as a differential diagnosis or concomitant condition.
The most frequently used scales in practice are the Hamilton–Norwood scale (Fig. 1) for male pattern distribution and the Ludwig scale (Fig. 2) or Olsen scale (Fig. 4) (frontal accentuation/Christmas tree pattern) for a female pattern. The experts agreed to use these scales in practice. The 5-point Sinclair scale (Fig. 3) is more complicated than the Ludwig scale but offers a broader range of categories. This could become important as more and more women consult their doctor in the early stages of hair loss.[36]
A recent publication described a new classification called BASP (basic and specific), which is based on the pattern of hair loss, including the shape of the frontal hair line and the hair density in the frontal and the vertex areas.[38]
Facial and Body Hair Look for abnormal facial and body hair density and/or distribution. Absent or reduced eyebrows/eyelashes and/or body hair may suggest alopecia areata. Think also of frontal fibrosing alopecia if eyebrows or eyelashes are absent or reduced. The experts stated that some women with AGA also complain of reduction of eyebrows or eyelashes.
If there is excessive terminal body hair growth, examine the distribution. Think of ethnic hypertrichosis, hypertrichosis due to medications or hirsutism. Look for signs of acne, seborrhoea and obesity, which are clues for hyperandrogenism.
Nail Examination Nail abnormalities are not typical for AGA, but occur in alopecia areata, certain deficiencies and lichen planus.
Laboratory
Measurement of ferritin level or thyroid-stimulating hormone may be considered depending on the individual history, especially in diffuse effluvium. The role of adequate serum ferritin levels during treatment of diffuse androgen-dependent alopecia in women has been suggested by Rushton and Ramsay[39] and Kantor et al.,[40] who reported a significantly lower mean ferritin level in women with AGA compared with controls. Since then, the relationship between iron deficiency and hair loss has been debated and reviewed recently by Trost et al.,[41] who found that it was still insufficient evidence to recommend supplementation in the absence of insufficient deficiency anaemia. More recently, Bregy and Trüeb[42] suggested no association between serum ferritin levels > 10 μg L−1 and hair loss in women.
Men In men, laboratory testing for the diagnosis of AGA is unnecessary, except if the history or examination provide clues for another underlying disorder or associated disease.
Note: Additional laboratory examinations may become necessary before starting specific treatments. Therefore, the Consensus Group agreed that for men above 45 years measuring the prostate-specific antigen (PSA) value should be recommended before starting finasteride therapy, albeit PSA is controversially discussed. Nevertheless, finasteride reduces PSA values and can delay diagnosis or mask detection of prostate cancer. The treating urologist should be informed on the patient's finasteride intake.
Women The experts agreed that an extensive endocrinological work-up is not necessary. An interdisciplinary approach involving gynaecologists, endocrinologists and dermatologists is recommended if the history and clinical examination are indicative of androgen excess [e.g. polycystic ovary syndrome (anovulatory cycle, elevated hormonal levels), cycle disturbances, androgen-secreting tumours]. The group agreed to perform a free androgen index test [FAI = total testosterone (nmol L−1) × 100/sex hormone-binding globulin (SHBG) (nmol L−1)] and prolactin as screening parameters.[30] Depending on the results, further endocrinological investigations may be required. Free testosterone and FAI seem to be sensitive for the detection of hyperandrogenaemia. In women, at least 80% of bound serum testosterone is bound to SHBG. Consequently, free serum testosterone levels are substantially influenced by SHBG levels, which limit the interpretation of free serum testosterone. The FAI takes this SHBG dependence into account. FAI levels of 5 and above are indicative for polycystic ovary syndrome.[43] Other disorders presenting with clinical and/or biochemical signs of hyperandrogenism such as congenital adrenal hyperplasia, androgen-secreting tumours or Cushing syndrome should be excluded. For this purpose further laboratory testing, e.g. 17-OH-progesterone, follicle-stimulating hormone, oestradiol, prolactin or cortisol may be necessary.
Note: It makes sense to take any hormonal level only on the condition that there is no hormonal intake. Oestrogens lead to elevated SHBG levels, whereas testosterone levels may be only slightly changed. Consequently, the FAI can be markedly improved by hormonal contraception.[44] Therefore, the minimum pause in hormonal contraception has to be 2 months. The measurements should be taken between 08.00 and 09.00 h, ideally between the second and fifth days of the menstrual cycle.
Children and Adolescents In children and adolescents with premature onset of AGA an interdisciplinary approach between the dermatologist and paediatric endocrinologist should be taken.
Diagnostic Techniques and Clinical Documentation
Pull Test
The pull test is an examination that is easy to perform and to repeat, to roughly judge active hair shedding. Briefly, 50–60 hairs are grasped by thumb, index and middle fingers. While the hairs are tugged away, the fingers slide along the hair shaft. The pull test is positive when more than 10% of the grasped hair can be pulled out.[36] The test has a large inter-observer variation and is influenced by shampooing so that each examiner should personally standardize their own procedure. The pull test should be performed in the right and left parietal, frontal and occipital regions and in the visibly affected areas, as shortening of the cycle increases telogen rates and the pull test becomes positive. The pull test in different scalp regions is useful in excluding diffuse effluvium.[36]
In patients with AGA the pull test is positive only in the active phase with increased telogen hairs in the affected area. It may be frontally accentuated or diffusely positive. A diffuse positive pull test requires further diagnostic tests to exclude diffuse telogen effluvium.
The pull test is usually negative in AGA, except in active periods when there can be a moderate telogen hair shedding in a pattern distribution. However, even with a diffusely positive pull test such as in telogen effluvium or diffuse alopecia areata, underlying AGA may be present.
Dermoscopy or Loupe
Dermoscopy or loupe are noninvasive techniques, which improve examination of scalp skin and hair by magnification, e.g. assessment of hair follicle openings to exclude scarring alopecia is facilitated. In combination with video mounting (videodermoscopy) the storing of diagnostic findings for further controls can be taken into account.[36,45]
In AGA increased hair diameter diversity and an increased number of vellus hairs can be seen. Less common are peripilar signs, reflecting the presence of perifollicular infiltrates, and yellow dots more prevalent in alopecia areata.[35]
Global Photography
Global photographs are helpful tools to evaluate objectively the course of hair growth, hair volume and hair density in clinical studies and for long-term follow-up in daily practice. In clinical studies the photographs of vertex, mid-pattern, frontal and temporal regions are standardized by using a stereotactic device assuring a constant view, magnification and lightening for follow-up assessment.[46] It was agreed that global photography is suitable for daily clinical practice only when a standardized technique is used.
Automatic Digitalized System for Hair Density and Anagen/Telogen Hairs (Phototrichogram/Trichoscan)
These systems for measurement of hair density and the anagen/telogen ratio can be used for diagnosis and follow-up, where available, but their use is mainly as a tool for clinical studies. In AGA the frontal hair density is decreased compared with the occipital density. The anagen/telogen ratio is normal or decreased.[47] To ensure reproducibility, tattoos identifying the area are required.[36] These techniques are helpful for long-term follow-up and quantification.[48,49]
Trichogram
The trichogram is indicated only in individual cases where a loose anagen syndrome or anagen-dysplastic effluvium is suspected. A trichogram should be used only by dermatologists who are familiar with this technique and perform it routinely.[50]
Biopsy
The scalp biopsy is an essential instrument in the diagnosis of cicatricial and selected forms of noncicatricial alopecia.[51] A biopsy, mostly performed as a 4-mm cylindrical punch, is indicated in AGA only in cases where the diagnosis is uncertain, e.g. where scalp changes suggestive of cicatricial alopecia or diffuse alopecia areata are present. Scalp biopsies should be reported by dermatopathologists who are experienced in hair pathology using both vertical and horizontal sectioning.
Site of Biopsy The preferred area for biopsy is the central scalp in an area representative of the hair loss process. Biopsies should not be taken from the bitemporal area as this region may have miniaturized hairs independent of AGA.
Type of Biopsy Two 4-mm punch biopsies following the direction of the hair shafts are taken lateral to the midline deep into the subcutaneous fat where anagen hair bulbs are located. One biopsy is processed for conventional vertical sectioning; the other is sectioned horizontally with respect to the skin surface.[51–53]
Horizontal sectioning allows a rapid assessment of hair follicle numbers, diameter, grouping and morphology.[54] In AGA, there is an increased number and proportion of miniaturized (vellus-like) hair follicles. The ratio of terminal to vellus-like hair follicles is typically < 3 : 1 in AGA-affected areas compared with > 7 : 1 in the normal scalp.[53] Other features include an increased telogen : anagen ratio and an increase in the number of follicular stelae (tracts beneath miniaturized follicles).[55] A mild perifollicular lymphohistiocytic infiltration primarily around the upper follicle as well as perifollicular fibrosis may also be seen.[20]
Summary
Generally, AGA is a clinical diagnosis. Depending on patient history and clinical evaluation, further diagnostic testing may be necessary. To facilitate implementation of the S1 guideline in daily clinical routine, the expert opinion-based recommendations of the current guideline are summarized in a two-page diagnostic evaluation form. The latter needs only a few minutes to be completed. It is a checklist containing all essential issues to diagnose AGA. Moreover, the Consensus Group developed a clinical algorithm that summarizes the steps in the diagnostic procedure.
Guideline for Diagnostic Evaluation in Androgenetic Alopecia in Men, Women and Adolescents
U. Blume-Peytavi; A. Blumeyer; A. Tosti; A. Finner; V. Marmol; M. Trakatelli; P. Reygagne; A. Messenger
Posted: 01/12/2011; The British Journal of Dermatology. 2011;164(1):5-15. © 2011 Blackwell Publishing
Abstract and Introduction
Abstract
Androgenetic alopecia (AGA) is the most common hair loss disorder, affecting both men and women. Due to the frequency and the often significant impairment of life perceived by the affected patients, competent advice, diagnosis and treatment is particularly important. As evidence-based guidelines on hair disorders are rare, a European consensus group was constituted to develop guidelines for the diagnostic evaluation and treatment of AGA. This S1 guideline for diagnostic evaluation of AGA in men, women and adolescents reviews the definition of AGA and presents expert opinion-based recommendations for sex-dependent steps in the diagnostic procedure.
Introduction
Evidence-based guidelines on hair disorders are rare, except for one S2 guideline on alopecia areata by the British Association of Dermatologists.[1] No national, European or international guidelines have been established for the diagnosis and treatment of androgenetic alopecia (AGA). Three different types of evidence-based guidelines (types S1–S3) exist. An S1 guideline is built by an informal consensus of an expert group. The statements for S2 guidelines are formed by a formal consensus process. An S3 guideline is based on a consensus from a systematic literature research with evaluation of evidence levels and a systematic decision process.
A European consensus group was built, consisting of members from different countries, organizations, specialities and interest groups. A detailed literature search on diagnosis and treatment of AGA using Medline, Embase, Cochrane and a hand search was performed. Based on the literature available the group decided to undergo an informal consensus process on an S1 level for the diagnosis of AGA and an S3 guideline process for the therapy of AGA (published separately). A subgroup of this European Consensus Group consisting of the eight authors of this article decided to work on this S1 guideline which was funded by the Verein Pro Haut e.V. Berlin, and is therefore independent and without any commercial conflict of interest.
The European Consensus Group reviewed the definition of AGA and established a consensus for the diagnosis of AGA dealing with the following points: expert opinion-based recommendation for the diagnosis of AGA in female and male patients, as well as in adolescents. The aim was to develop a diagnostic evaluation form and recommendations for diagnostic procedures to assist in the daily work of the practitioner. The questionnaire for daily practice was kept simple and it is planned to be validated during consultation by practising dermatologists experienced in the management of hair disorders.
Definition of Androgenetic Alopecia
AGA is a nonscarring progressive miniaturization of the hair follicle with a usually characteristic pattern distribution in genetically predisposed men and women. In men, AGA typically shows a pattern distribution, most commonly a male pattern, but occasionally a female pattern can be seen.[2,3] In women, AGA typically presents as a diffuse reduction in hair density over the frontal and central areas, but the parietal and occipital regions may also be involved.[4] Occasionally, AGA occurs in a male pattern in women.
Differential Diagnoses and Aggravating Factors of Androgenetic Alopecia
The diagnosis of AGA is normally made clinically, but aggravating associated factors and other diseases affecting scalp and hair growth need to be excluded. As certain treatments (e.g. finasteride) are selectively effective in AGA, it is important to rule out other hair loss disorders resembling pattern hair loss.
Frequency and Prevalence
Men
Male AGA occurs in all populations. The prevalence is highest in Caucasians, reaching around 80% in men aged over 70 years.[5,6] In the Asian population, a prevalence of 46·9–60·0% has been reported in males older than 70 years.[3,7] There is scant published information on the frequency of balding in African men. One older study reported that balding is four times less common in African-American men than in Caucasians. The frequency and severity of male AGA increase with age in all ethnic groups.[2,3] Initial signs of AGA, including some recession of the frontal hair line and at the temples, usually develop during teenage years. Progression to deep frontal recession and/or vertex balding may also start shortly after puberty, although in most men the onset is later. By the age of 70 years, 50–60% of Caucasian men are bald (Hamilton–Norwood VI–VII).[2,6]
Women
As in men, the population frequency and severity of AGA increase with age in women.[5] Two studies in Caucasian women in the U.K. and the U.S.A. reported prevalence rates of 3–6% in women aged under 30, increasing to 29–42% in women aged 70 years and over.[8,9] The frequency is lower in Oriental women compared with those of European descent.[3] There are no published data on the frequency of AGA in African women.
Aetiology
Men
AGA is an androgen-dependent trait, which leads to progressive miniaturization of the hair follicle in predisposed men.[10] It is thought that enhanced androgen effects at the genetically predisposed hair follicles are mediated by raised androgen receptor density and/or increased activity of 5-alpha-reductase type II.[2] Nearly all men afflicted with AGA have normal circulating androgen levels. The predisposition to AGA is predominately due to genetic factors. Twin studies show strong concordance rates of between 80% and 90% for monozygotic twins. Family analyses show a significantly increased risk for the development of AGA in men with a father suffering from AGA.[11,12] Conversely, the risk of AGA is significantly decreased in those men with a nonbalding father.
The current scientific data support the thesis that AGA has a polygenic trait. Significant associations have been reported with variant regions of the androgen receptor gene, which is located on the X-chromosome, using a candidate gene approach.[13,14] More recently, two independent genome-wide association studies have identified a susceptibility locus at chromosome 20p11.[15,16]
Women
There are few studies on the genetic base of AGA in women. Smith and Wells[12] reported the frequency of female pattern hair loss with an incidence of 54% pattern hair loss in first-degree male relatives aged > 30 years and 21% in first-degree female relatives > 30 years. It is possible that early- and late-onset female AGA are genetically distinct entities. The role of androgens in female AGA is less certain than in men and other factors may be involved. For the purpose of this guideline the term 'androgenetic alopecia' is used, although the uncertainty of the role of androgens is recognized.
The European Consensus Group agreed not to differentiate between androgenic alopecia and AGA, but to define a subset of women with AGA and associated hormonal dysregulation.
Clinical Picture
Men
In most men, AGA involves the fronto temporal area and the vertex, following a pattern corresponding to the Hamilton–Norwood scale (Fig. 1).[17,18] In some instances, however, men develop diffuse thinning of the crown with retention of the frontal hairline with a pattern that resembles the Ludwig type observed in women.
Women
Female pattern hair loss may have three different patterns:
1.Diffuse thinning of the crown region with preservation of the frontal hairline. There are two scales that describe this pattern: the commonly used 3-point Ludwig scale (Fig. 2)[19,20] and the 5-point Sinclair scale (Fig. 3).[21–23]
2.Thinning and widening of the central part of the scalp with breach of frontal hairline (Olsen scale: Christmas tree pattern, Fig. 4).[20,24]
3.Thinning associated with bitemporal recession (Hamilton–Norwood type, Fig. 1).[17,18,24]
However, involvement of the parietal and occipital scalp with diffuse alopecia is clinically observed (Fig. 5).[24,25]
Steps in the Diagnostic Procedure
The European Consensus Group developed a diagnostic evaluation form (Table 1) for AGA, including history, clinical evaluation, diagnostic techniques and clinical documentation. This form can easily be implemented in daily clinical routine. Additionally, the information has been summarized in a clinical algorithm (Fig. 6).
DD, differential diagnosis; DHEAS, dehydroepiandrosterone sulphate; FAI, free androgen index test; RPR, rapid plasma regain; SHBG, sex hormone-binding globulin; TPHA, treponemal haemagglutinin assay; TSH, thyroid-stimulating hormone.
Figure 6. Clinical algorithm for the diagnosis of androgenetic alopecia (AGA). DD, differential diagnosis.

General The physician should record age, sex, age at first manifestation of hair loss and course of hair loss (chronic or intermittent) in the personal as well as in the family history. Patients with AGA usually complain about a long-standing, slowly progressing hair loss. The hair loss may be described as chronic, but the patients often report increased activity in autumn and winter. The patient should be asked about increased shedding or thinning or both. For thinning, the patient typically describes an accentuation of the frontal, parietal or vertex region, but complaints about diffuse thinning are possible as well. Initial signs of AGA can be itching and trichodynia. A family history for AGA is often positive, but a negative family history does not exclude the diagnosis. A positive family history of other hair disorders, such as alopecia areata or hirsutism may influence the extent of further diagnostic procedures. It is also important to determine if there are other concomitant hair disorders whose untreated presence could ultimately affect the efficacy of the treatments used for AGA (e.g. iron deficiency in diffuse hair loss in women).
A detailed history on systemic and newly diagnosed diseases within 1 year prior to first signs of hair loss should arouse suspicion that the hair loss is due to other causes or aggravating factors, such as diffuse effluvium as a result of severe infection, iron deficiency or thyroid dysfunction. Allergies are not important for the diagnosis of AGA, but they should be recorded for possible significance with regard to therapy (e.g. contact dermatitis due to propylene glycol in minoxidil solutions). The Consensus Group agreed to ask about eating behaviour, as chronic deficient diet or rapid significant weight loss can trigger diffuse effluvium. Concerning drug history there are a variety of drugs that can cause hair loss. Ask especially for chemotherapeutic agents (almost all of which can produce hair loss) and for hormones with pro androgenic or antithyroid action, and whether there is coincidence with hair loss. Particularly in men, the intake of anabolic steroids or supplemental androgens should be explored.
Moreover, consider lifestyle procedures such as special hairstyles (traction) and environmental factors like smoking and ultraviolet radiation (UVR) exposure in the history of the patient. Su and Chen[26] reported a dose-dependent association between smoking status and development of moderate to severe AGA in male patients. Additionally, UVR exposure as an aggravating factor should be taken into account, especially in Mediterranean countries.[27,28]
Women Although the majority of female patients with AGA have physiological hormonal function, an orienting gynaecological history should be done to exclude influencing hormonal dysregulations or associated underlying disorders (e.g. hormone-sensitive tumour). Careful evaluation of the gynaecological history should include menarche, menstrual cycle (regular/irregular), menopause, amenorrhoea, the use of oral or systemic hormonal contraception, hormone replacement therapy, fertility treatment, the number of pregnancies and births, time of last pregnancy/delivery, impaired fertility or problems in getting pregnant, miscarriages, gynaecological surgery, signs of hyperandrogenism (excessive body hair growth, skin changes like acne) or seborrhoea of scalp/skin. Irregular menstrual cycle, amenorrhoea, impaired fertility, problems in getting pregnant, miscarriages, signs of hyperandrogenism and seborrhoea may be indicative for hormonal dysregulation, such as polycystic ovary syndrome.[29–31] If the patient is taking hormonal contraception, ask for cycle duration and regularity before the intake of hormonal contraception.
Adolescents In adolescents it is first important to differentiate if the hair loss is congenital or acquired. Look for physical and mental development, particularly early onset of puberty. In childhood/adolescence, AGA is an acquired hair loss with a characteristic pattern distribution. The differential diagnosis includes diffuse effluvium (nutritional factors), artificially induced alopecia, hypotrichosis simplex (congenital) or ectodermal dysplasia (congenital, delayed physical/psychological development, sweating, dental or nail abnormalities). AGA without signs of premature puberty is not necessarily associated with endocrinological dysregulation, but referral to a paediatric endocrinologist is recommended.
Clinical Evaluation
Clinical examination should involve the scalp skin and hair, facial and body hair and skin as well as the nails.
Scalp Examination The scalp skin usually appears normal in AGA, but consider that seborrhoeic dermatitis of the scalp can be an associated and potentially aggravating factor.[6,32,33]
Look for signs of inflammation (erythema, scaling and hyperkeratosis), seborrhoea and signs of scarring (atrophy, loss of hair follicle ostia). Consider inflammatory or infectious diseases and tumours. Consider alopecia areata and scarring alopecias, which can mimic AGA, especially frontal fibrosing alopecia (Kossard[34]) in women. Look for signs of sun damage in balding areas, which may be an aggravating factor for AGA.[28] In long-standing AGA, discrete atrophy of scalp skin can be present.
Hair Examination Scalp hair Part the hair to assess scalp hair density. Compare part width in the frontal, occipital and temporal regions to examine the distribution of alopecia. On close examination, focal atrichia of a few millimetres in diameter may be present in some women with AGA. Use a sheet of paper over a part to enhance contrast and look for short and fine miniaturized or broken hairs, variation of hair calibre, length and regrowth. Dermoscopy may be helpful.[35] A pull test, Sabouraud manoeuvre and a friction test are simple tests to get a first global impression of hair quality and hair growth activity.[36,37]
Evaluate and distinguish between diffuse decrease in hair density, a characteristic pattern distribution or localized alopecic patches. In males, AGA typically presents with a male pattern distribution including bitemporal recession and/or vertex thinning, and sometimes anterior recession. About 10% of men with AGA present a female pattern.
Women usually show a more diffuse distribution of hair loss with accentuation in the frontal and mid scalp and preservation of the frontal hairline, but the parietal and occipital scalp may also be involved. In cases of diffuse thinning, also think of diffuse telogen effluvium and diffuse alopecia areata as a differential diagnosis or concomitant condition.
The most frequently used scales in practice are the Hamilton–Norwood scale (Fig. 1) for male pattern distribution and the Ludwig scale (Fig. 2) or Olsen scale (Fig. 4) (frontal accentuation/Christmas tree pattern) for a female pattern. The experts agreed to use these scales in practice. The 5-point Sinclair scale (Fig. 3) is more complicated than the Ludwig scale but offers a broader range of categories. This could become important as more and more women consult their doctor in the early stages of hair loss.[36]
A recent publication described a new classification called BASP (basic and specific), which is based on the pattern of hair loss, including the shape of the frontal hair line and the hair density in the frontal and the vertex areas.[38]
Facial and Body Hair Look for abnormal facial and body hair density and/or distribution. Absent or reduced eyebrows/eyelashes and/or body hair may suggest alopecia areata. Think also of frontal fibrosing alopecia if eyebrows or eyelashes are absent or reduced. The experts stated that some women with AGA also complain of reduction of eyebrows or eyelashes.
If there is excessive terminal body hair growth, examine the distribution. Think of ethnic hypertrichosis, hypertrichosis due to medications or hirsutism. Look for signs of acne, seborrhoea and obesity, which are clues for hyperandrogenism.
Nail Examination Nail abnormalities are not typical for AGA, but occur in alopecia areata, certain deficiencies and lichen planus.
Laboratory
Measurement of ferritin level or thyroid-stimulating hormone may be considered depending on the individual history, especially in diffuse effluvium. The role of adequate serum ferritin levels during treatment of diffuse androgen-dependent alopecia in women has been suggested by Rushton and Ramsay[39] and Kantor et al.,[40] who reported a significantly lower mean ferritin level in women with AGA compared with controls. Since then, the relationship between iron deficiency and hair loss has been debated and reviewed recently by Trost et al.,[41] who found that it was still insufficient evidence to recommend supplementation in the absence of insufficient deficiency anaemia. More recently, Bregy and Trüeb[42] suggested no association between serum ferritin levels > 10 μg L−1 and hair loss in women.
Men In men, laboratory testing for the diagnosis of AGA is unnecessary, except if the history or examination provide clues for another underlying disorder or associated disease.
Note: Additional laboratory examinations may become necessary before starting specific treatments. Therefore, the Consensus Group agreed that for men above 45 years measuring the prostate-specific antigen (PSA) value should be recommended before starting finasteride therapy, albeit PSA is controversially discussed. Nevertheless, finasteride reduces PSA values and can delay diagnosis or mask detection of prostate cancer. The treating urologist should be informed on the patient's finasteride intake.
Women The experts agreed that an extensive endocrinological work-up is not necessary. An interdisciplinary approach involving gynaecologists, endocrinologists and dermatologists is recommended if the history and clinical examination are indicative of androgen excess [e.g. polycystic ovary syndrome (anovulatory cycle, elevated hormonal levels), cycle disturbances, androgen-secreting tumours]. The group agreed to perform a free androgen index test [FAI = total testosterone (nmol L−1) × 100/sex hormone-binding globulin (SHBG) (nmol L−1)] and prolactin as screening parameters.[30] Depending on the results, further endocrinological investigations may be required. Free testosterone and FAI seem to be sensitive for the detection of hyperandrogenaemia. In women, at least 80% of bound serum testosterone is bound to SHBG. Consequently, free serum testosterone levels are substantially influenced by SHBG levels, which limit the interpretation of free serum testosterone. The FAI takes this SHBG dependence into account. FAI levels of 5 and above are indicative for polycystic ovary syndrome.[43] Other disorders presenting with clinical and/or biochemical signs of hyperandrogenism such as congenital adrenal hyperplasia, androgen-secreting tumours or Cushing syndrome should be excluded. For this purpose further laboratory testing, e.g. 17-OH-progesterone, follicle-stimulating hormone, oestradiol, prolactin or cortisol may be necessary.
Note: It makes sense to take any hormonal level only on the condition that there is no hormonal intake. Oestrogens lead to elevated SHBG levels, whereas testosterone levels may be only slightly changed. Consequently, the FAI can be markedly improved by hormonal contraception.[44] Therefore, the minimum pause in hormonal contraception has to be 2 months. The measurements should be taken between 08.00 and 09.00 h, ideally between the second and fifth days of the menstrual cycle.
Children and Adolescents In children and adolescents with premature onset of AGA an interdisciplinary approach between the dermatologist and paediatric endocrinologist should be taken.
Diagnostic Techniques and Clinical Documentation
Pull Test
The pull test is an examination that is easy to perform and to repeat, to roughly judge active hair shedding. Briefly, 50–60 hairs are grasped by thumb, index and middle fingers. While the hairs are tugged away, the fingers slide along the hair shaft. The pull test is positive when more than 10% of the grasped hair can be pulled out.[36] The test has a large inter-observer variation and is influenced by shampooing so that each examiner should personally standardize their own procedure. The pull test should be performed in the right and left parietal, frontal and occipital regions and in the visibly affected areas, as shortening of the cycle increases telogen rates and the pull test becomes positive. The pull test in different scalp regions is useful in excluding diffuse effluvium.[36]
In patients with AGA the pull test is positive only in the active phase with increased telogen hairs in the affected area. It may be frontally accentuated or diffusely positive. A diffuse positive pull test requires further diagnostic tests to exclude diffuse telogen effluvium.
The pull test is usually negative in AGA, except in active periods when there can be a moderate telogen hair shedding in a pattern distribution. However, even with a diffusely positive pull test such as in telogen effluvium or diffuse alopecia areata, underlying AGA may be present.
Dermoscopy or Loupe
Dermoscopy or loupe are noninvasive techniques, which improve examination of scalp skin and hair by magnification, e.g. assessment of hair follicle openings to exclude scarring alopecia is facilitated. In combination with video mounting (videodermoscopy) the storing of diagnostic findings for further controls can be taken into account.[36,45]
In AGA increased hair diameter diversity and an increased number of vellus hairs can be seen. Less common are peripilar signs, reflecting the presence of perifollicular infiltrates, and yellow dots more prevalent in alopecia areata.[35]
Global Photography
Global photographs are helpful tools to evaluate objectively the course of hair growth, hair volume and hair density in clinical studies and for long-term follow-up in daily practice. In clinical studies the photographs of vertex, mid-pattern, frontal and temporal regions are standardized by using a stereotactic device assuring a constant view, magnification and lightening for follow-up assessment.[46] It was agreed that global photography is suitable for daily clinical practice only when a standardized technique is used.
Automatic Digitalized System for Hair Density and Anagen/Telogen Hairs (Phototrichogram/Trichoscan)
These systems for measurement of hair density and the anagen/telogen ratio can be used for diagnosis and follow-up, where available, but their use is mainly as a tool for clinical studies. In AGA the frontal hair density is decreased compared with the occipital density. The anagen/telogen ratio is normal or decreased.[47] To ensure reproducibility, tattoos identifying the area are required.[36] These techniques are helpful for long-term follow-up and quantification.[48,49]
Trichogram
The trichogram is indicated only in individual cases where a loose anagen syndrome or anagen-dysplastic effluvium is suspected. A trichogram should be used only by dermatologists who are familiar with this technique and perform it routinely.[50]
Biopsy
The scalp biopsy is an essential instrument in the diagnosis of cicatricial and selected forms of noncicatricial alopecia.[51] A biopsy, mostly performed as a 4-mm cylindrical punch, is indicated in AGA only in cases where the diagnosis is uncertain, e.g. where scalp changes suggestive of cicatricial alopecia or diffuse alopecia areata are present. Scalp biopsies should be reported by dermatopathologists who are experienced in hair pathology using both vertical and horizontal sectioning.
Site of Biopsy The preferred area for biopsy is the central scalp in an area representative of the hair loss process. Biopsies should not be taken from the bitemporal area as this region may have miniaturized hairs independent of AGA.
Type of Biopsy Two 4-mm punch biopsies following the direction of the hair shafts are taken lateral to the midline deep into the subcutaneous fat where anagen hair bulbs are located. One biopsy is processed for conventional vertical sectioning; the other is sectioned horizontally with respect to the skin surface.[51–53]
Horizontal sectioning allows a rapid assessment of hair follicle numbers, diameter, grouping and morphology.[54] In AGA, there is an increased number and proportion of miniaturized (vellus-like) hair follicles. The ratio of terminal to vellus-like hair follicles is typically < 3 : 1 in AGA-affected areas compared with > 7 : 1 in the normal scalp.[53] Other features include an increased telogen : anagen ratio and an increase in the number of follicular stelae (tracts beneath miniaturized follicles).[55] A mild perifollicular lymphohistiocytic infiltration primarily around the upper follicle as well as perifollicular fibrosis may also be seen.[20]
Summary
Generally, AGA is a clinical diagnosis. Depending on patient history and clinical evaluation, further diagnostic testing may be necessary. To facilitate implementation of the S1 guideline in daily clinical routine, the expert opinion-based recommendations of the current guideline are summarized in a two-page diagnostic evaluation form. The latter needs only a few minutes to be completed. It is a checklist containing all essential issues to diagnose AGA. Moreover, the Consensus Group developed a clinical algorithm that summarizes the steps in the diagnostic procedure.